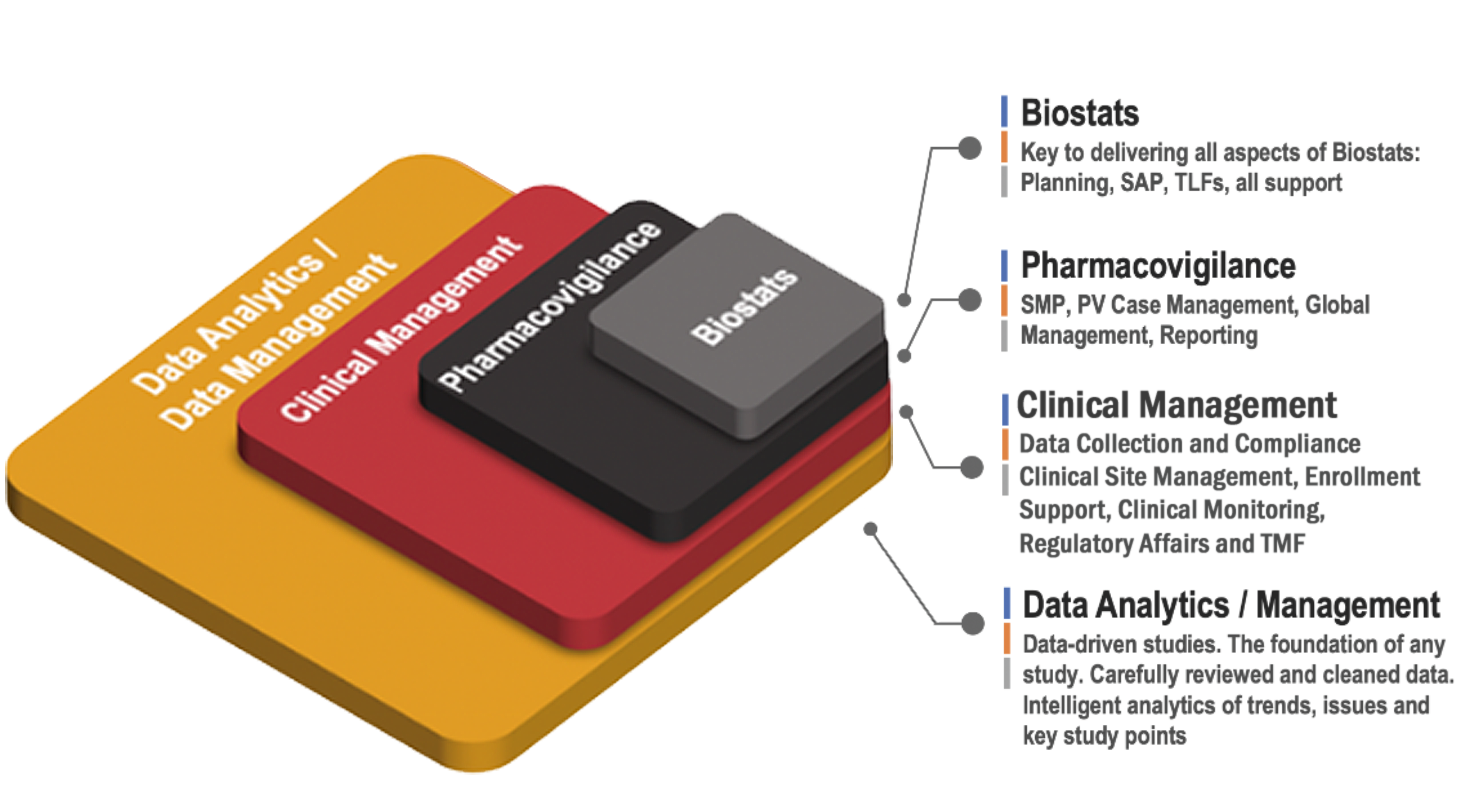
Data-Driven Studies:
Faster and smarter results delivered.
Power your next study with Axiom’s Fusion eClinical Suite and DM/PV/BioStats Services, the most configurable unified eClinical solution dedicated to small to medium sized medical device, pharma, biotech companies, and CROs worldwide.
Solutions We Bring To The Table:
• Standard Trials
• Virtual Trials
• Remote Trials
• Home Healthcare Supported Trials
• Rescue Projects & Database Migrations
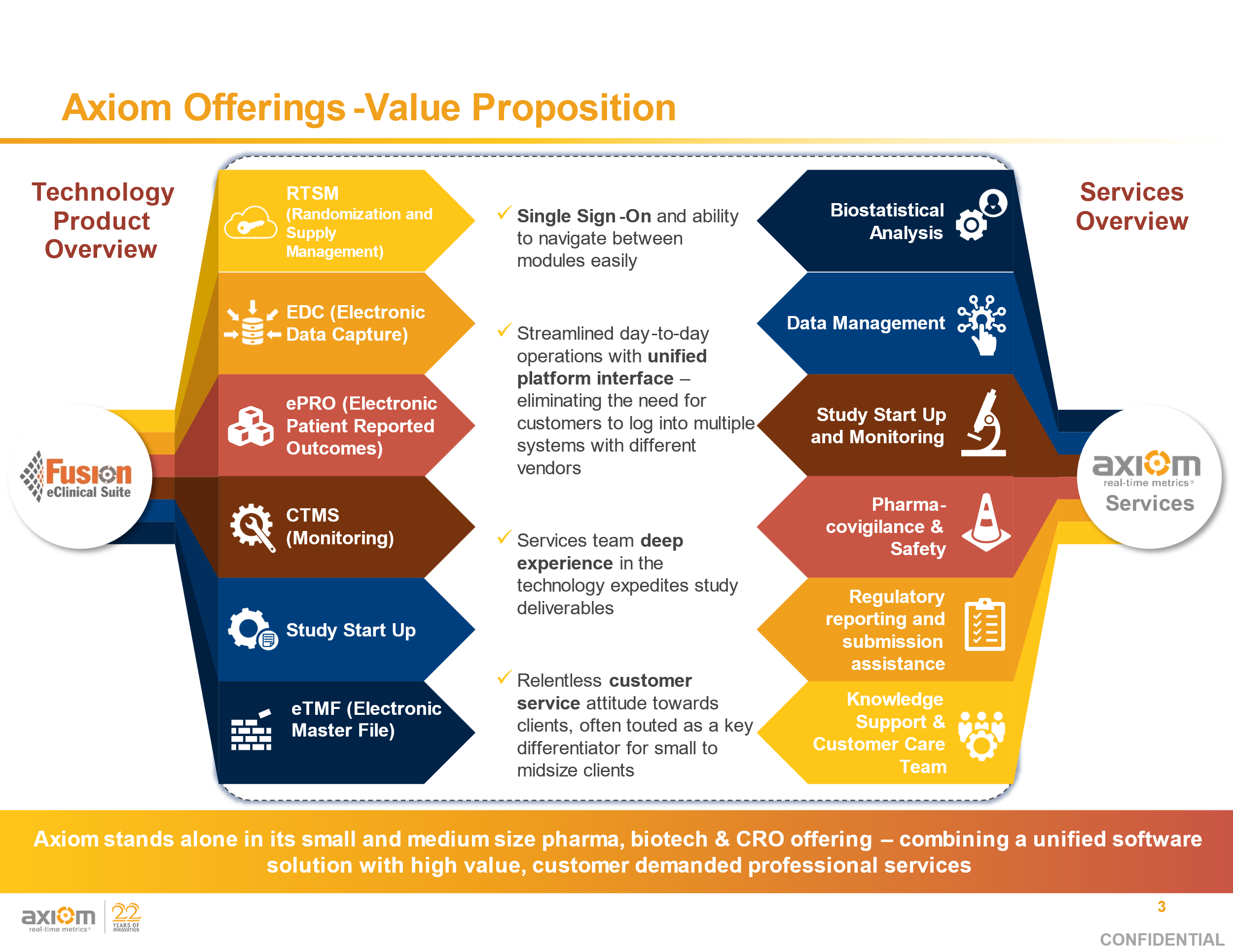
UNIFIED MODULES: CTM Tracking, Adjudication, IRT/Predictive Re-Supply, Image Import, AE/SAE/SADE Module, Safety Module, CTMS Module, ePRO, Randomization
MODULE REPORTS: Hand-Carry Tracking, Case Narrative Listings, SAE/SADE Management, Enrollment Tracking, Data Dashboards
SERVICES: Data Management, Pharmacovigilance, Clinical Science, Project Management, Medical Monitoring
KEY ASPECTS: Hand-Carry Management, Adjudication Oversight, Adjudicator Resourcing Tools
UNIFIED MODULES: CTM Tracking, Randomization, IRT/Predictive Re-Supply, Central Lab Import, AE/SAE Module, Safety Module, CTMS Module
MODULE REPORTS: IP Lifecycle Report, Case Narrative Listings, SAE Management, Enrollment Tracking, Data Dashboards
SERVICES: Data Management, Pharmacovigilance, Clinical Science, Project Management, Medical Monitoring
KEY DATA POINTS: Patient Profile Reports, Biomarker Tracking, Response Assessments
Deliver your studies faster, easier, and smarter with Axiom as your embedded partner.
From small Phase I studies, to global trials and registries, Axiom delivers 360 degree eClinical solutions and services to meet your clinical research needs. Connect with us to learn how we help you deliver your studies faster, easier and smarter.
 Explore Axiom's Professional Services
Explore Axiom's Professional ServicesAxiom is a Proud Member of:
“We received a quote for stand-alone EDC from a large technology vendor and were shocked. With Axiom we could resource our entire project – EDC, DM, safety, randomization, drug supply tracking and more – for significantly less that they quoted for data capture only. Our decision was easy.”Clinical Director, Boston-based biotech
“Axiom delivers the complete package for our device studies, eCRF design, EDC/DM solutions and services, real-time reporting, SAE/MedWatch forms generation, device supply and accountability and CEC/DSMB reporting. Thousands of hours saved over 12 months. A pleasure to work with.”Clinical Trials Manager, San Diego-based Device Firm
Why work with Axiom?
Know More. Know It Sooner. Act Faster.
Technology should empower you to know more, know it sooner, and act faster. That’s what good technology does.

Live access to clinical data reports, DSMB reporting and data listings. Getting access to the status of your study shouldn’t require a PhD in computer science. Real-time study reports in 2 clicks.
Axiom’s unified eClinical solutions means your EDC, Randomization, Drug / Device Tracking,Adjudication, Imaging and SAE / Safety data is in the same place.

Key notifications about all of the information progress and issues within your study delivered 24/7 to your smartphone or e-mail.
Manage safety events in Fusion, no need for a separate safety database. How about managing your AE / SAEs / SADEs and overall safety reporting from within Fusion? Track events. Create narratives.
Fully manage your randomization within Fusion eClinical Suite. No need to configure a separate randomization system. Fully integrated with Inventory Management for complete digital management of your IWRS/Inventory.

Global awareness of inventory activities, balances, and issues as well as notifications of key events including inventory levels, shipments, requests and pending activities. Stop managing your drug or device accountability in multiple systems.
One of the keys to study success is the focus of the organization on the study details, consideration for planning and strategic thinking that goes into the initiation of the study. Axiom’s Think / Deploy / Launch process ensures that a well thought-out process is the basis for planning your study.

Axiom’s Customer Care team are extremely focused on ensuring that every single user in the study chain is supported throughout the entire study. Great support is linked to high-quality data. Support personnel who actually are trained on your study’s eCRF. Quick answers to end-user questions means that the study moves along quickly and efficiently.
Expanding the technology footprint could cost more in the short term, but can greatly enhance the overall study, your real-time knowledge, key business decisions and lower costs in the long term.






