Fusion eClinical Suite
Data Driven Studies: Fusion is Axiom’s proprietary easy-to-use and cost-effective eClinical solution that is focused on small to medium sized device, pharma, and biotech companies.
Over 15 fully integrated modules that connect seamlessly in one unified platform – accessible with a single sign-on.
Single Sign-On Access
Single sign-on access to all of your fully integrated Fusion modules.
Centralized Data
Get a centralized view of the critical data in your studies.
Highly Configurable
Your study is unique. Choose the modules and reports you need.

The Fusion Advantage
Better decisions. Shorter timelines.
Unified Technology. Unmatched Service.
Explore All Fusion Modules
Access all of your Fusion modules and study data with a single sign-on.

Choose the modules that meet the unique requirements of your study.
With Axiom’s Fusion eClinical Suite, you can add-on any Modules to meet your study requirements. Running a small study? Just choose the base configuration with EDC, DM and AE/SAE Tracking. Running something more complex? Include powerful Modules such as Inventory Tracking, RTSM, Safety Management to make the management of your study so much easier.
Eliminate huge amounts of manual reconciliation with Fusion’s Modular Configuration.

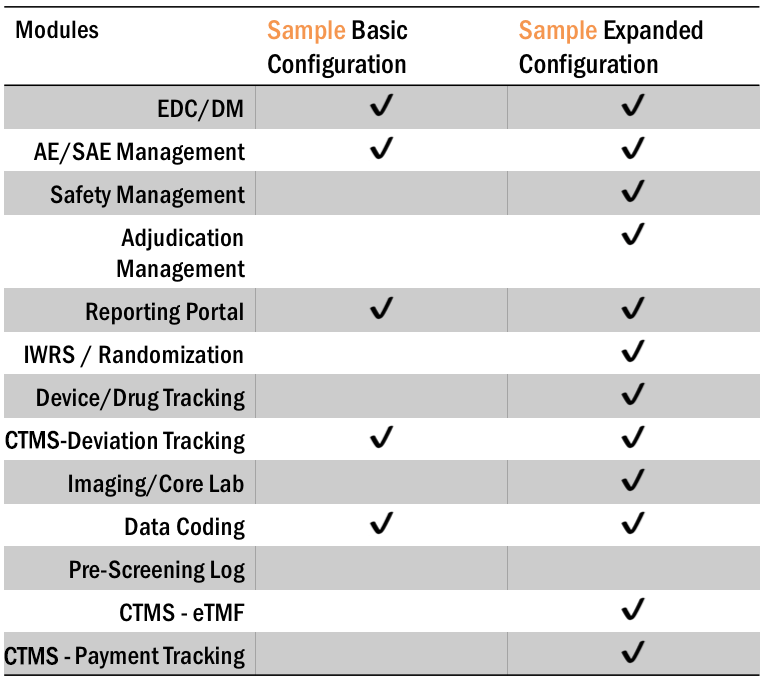 Fusion Pricing Model
Fusion Pricing ModelFusion eClinical Suite Modules
Choose from Fusion’s comprehensive suite of 15+ seamlessly integrated modules.
DATA ANALYTICS
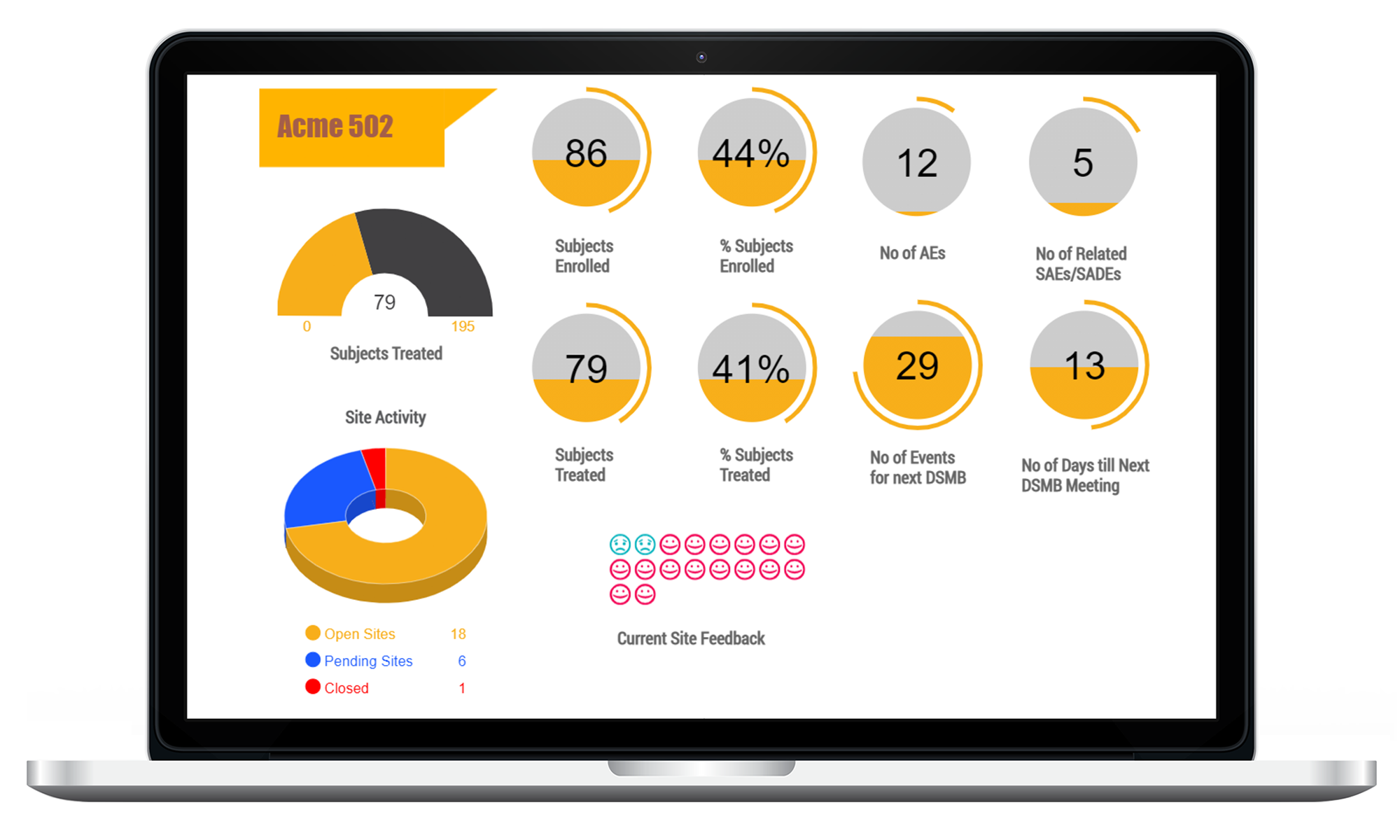
Access real-time data and reports across the global footprint of your study.
- Connector.
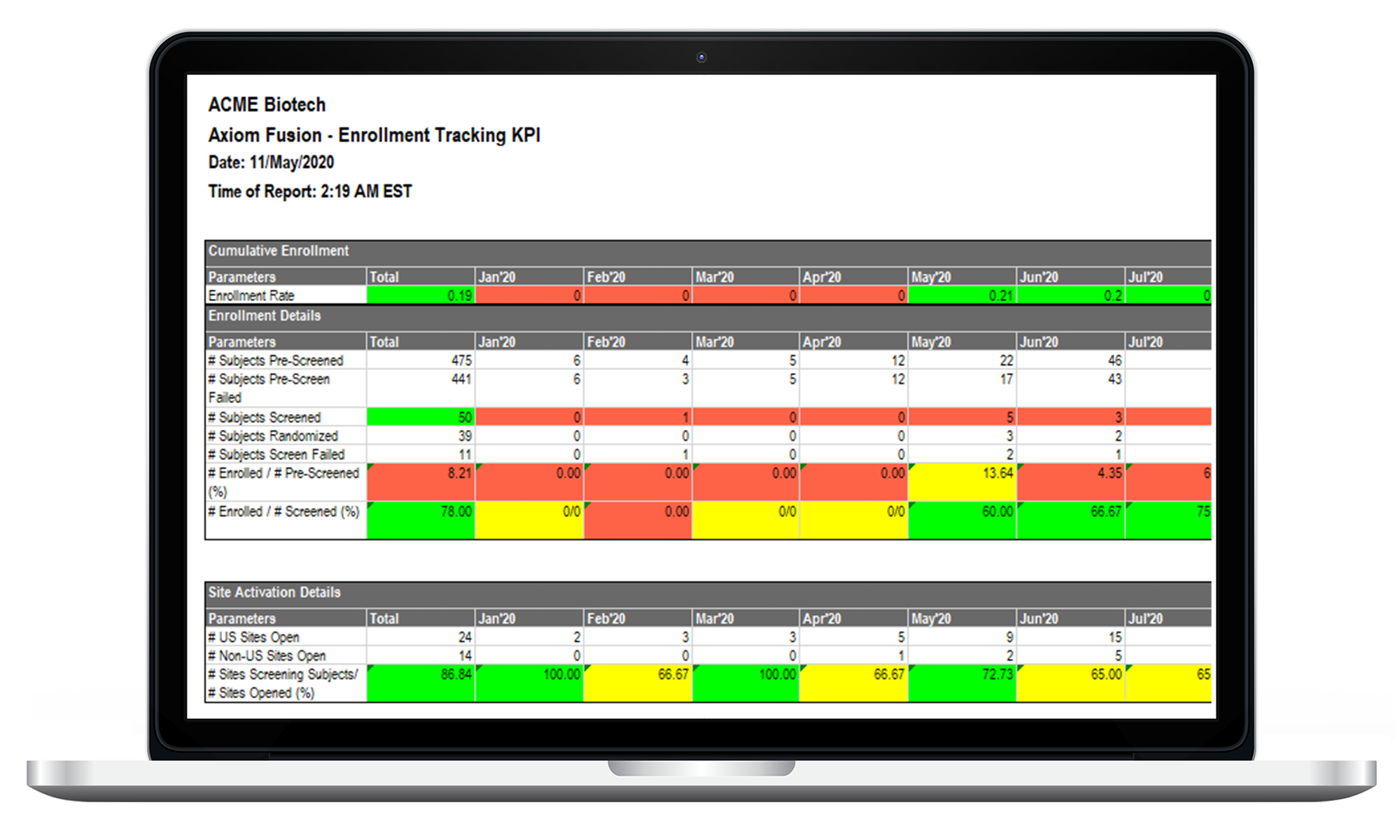
EDC / Data Management
Access real-time information access across the global footprint of your study. Eliminate manual processes and common data issues. LEARN MORE
- Connector.
eSource
Save time by capturing your study data electronically – even when offline! Data syncs to your Fusion databases once an internet connection is established. LEARN MORE
- Connector.
Pre-screening Log
Access real-time records for Subject screening activities.
- Connector.
Data Coding
Standardized data coding for your Fusion database.
- Connector.
General Log
Real-time access to your study’s general log.
- Connector.
Risk Management
Real-time dashboards for risk management.
ePRO / eCOA
Capture your ePRO/eCOA results using Axiom’s web, IVR or mobile device tools.
- Connector.
ePRO / eDiary Mobile App
Mobile app supports Bring Your Own Device (BYOD) and easy installation on iOS and Android. LEARN MORE
- Connector.
ePRO Patient Web Portal
Subjects can log in to Fusion Patient Web Portal to submit assessments on any browser. LEARN MORE
- Connector.
ePRO / eDiary Phone / IVRS
Call-in diary or questionnaire submissions using dial pad to select preset answers. LEARN MORE
- Connector.
eConsent
Enable Subjects to consent remotely.
RANDOMIZATION & TRIAL SUPPLY MANAGEMENT
Randomization and inventory management and reporting controlled via a single platform.
- Connector.
IWRS / RTSM / Cohort Management
Randomization / cohort management and reporting controlled via a single platform. LEARN MORE
- Connector.
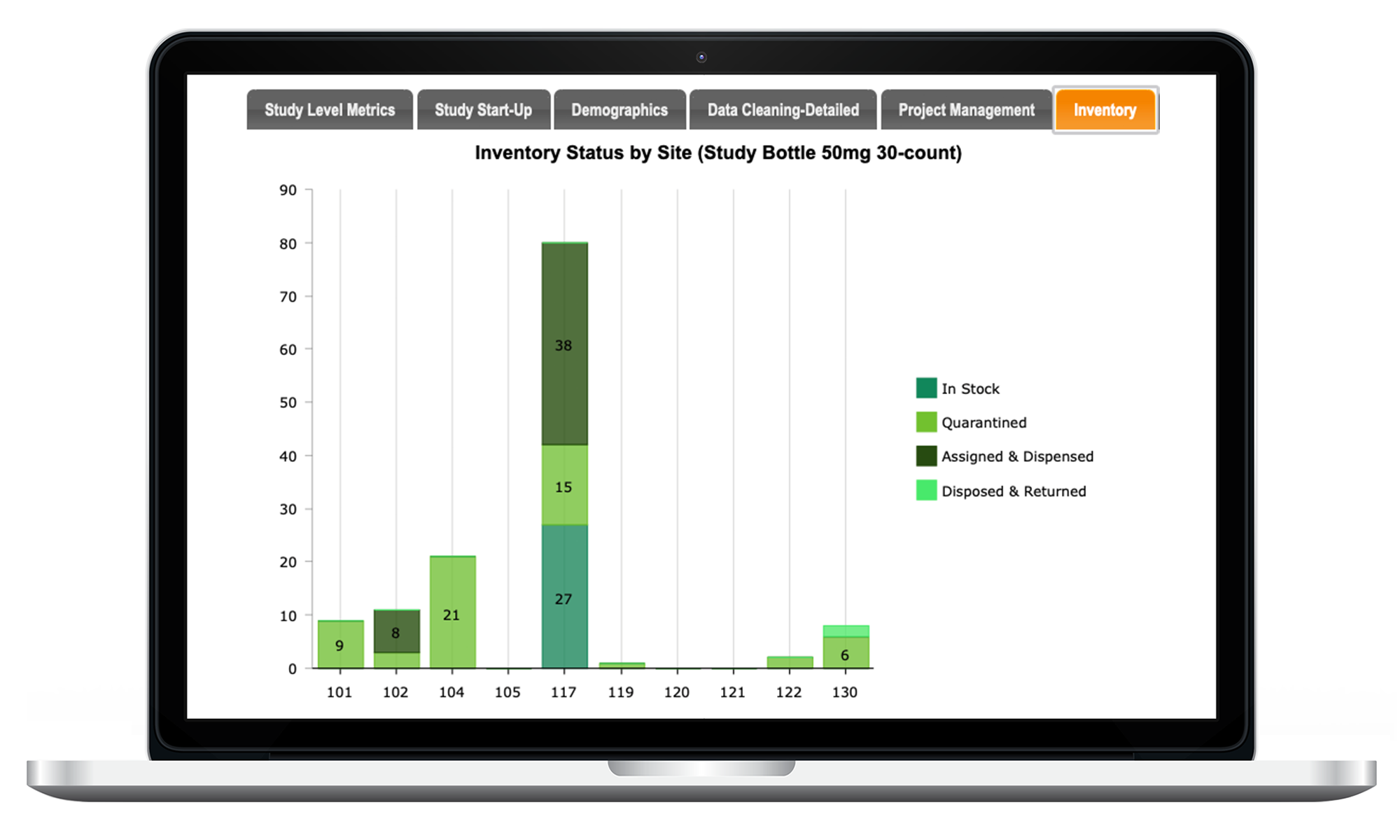
CTM Tracking / Inventory
Inventory / supply tracking and tasks are completely centralized and automated. LEARN MORE
SAFETY
Complete management and reporting of global safety events directly within Fusion.
- Connector.
AE / SAE Tracking
Fully integrated AE/SAE tracking and reporting for single or multi-country studies / regulatory bodies. LEARN MORE
- Connector.
Safety Module
Safety Database for the management of pharmacovigilance events directly within Fusion. LEARN MORE
CTMS / REPORTING
Standard and custom reports for every study. Track deviations, payments, trips and documents.
- Connector.
CTMS Dashboards / Reports
Highly customizable dashboards and over 75 real-time reports for viewing and export. LEARN MORE
- Connector.
Deviations Management
Centralized tracking, management and reporting of all site / study deviations. LEARN MORE
- Connector.
Payment Tracking
Fully integrated payment management and budgeting, without the need for an external solution. LEARN MORE
- Connector.
Monitor Visit Reporting
Book visits and approve requests. Enter data or upload reports with digital sign-off. LEARN MORE
- Connector.
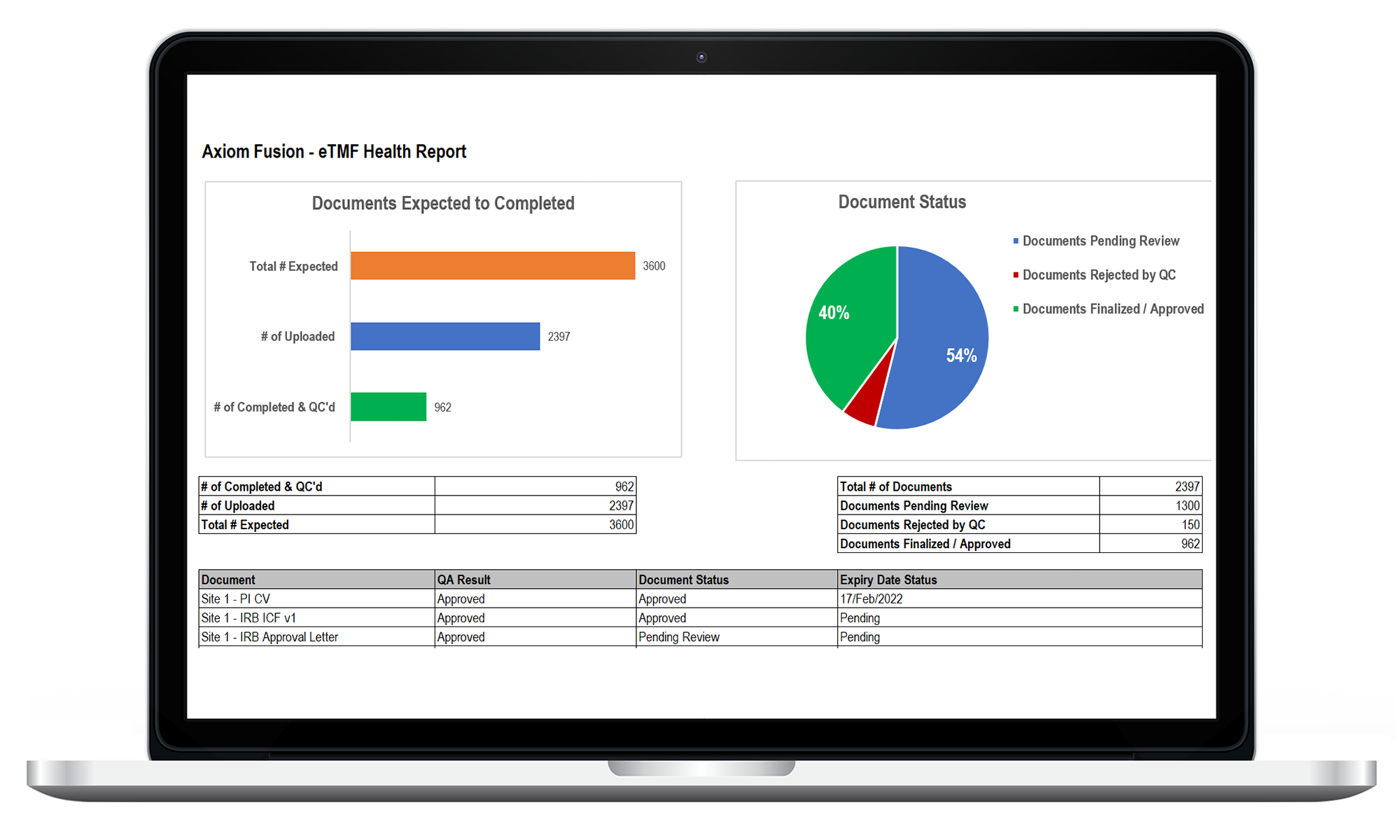
eTMF
Ensure your teams have secured, real-time access to all of your essential TMF documents. LEARN MORE
- Connector.
Study Start-Up
Start up your study in 30-60 days!
DATA IMPORT / ADJUDICATION
Seamlessly import data and files into Fusion. Manage adjudication without manual processes.
- Connector.
Central / Local Lab Import
Automatically import Excel data into Fusion for unified view and trends. LEARN MORE
- Connector.
Adjudication
Manage adjudication activities within Fusion without any manual processes. LEARN MORE
- Connector.
Imaging
Integrate your image requirements, and manage all your images through Fusion. LEARN MORE
- Connector.
Data Import
Easily import external study data into Fusion.
- Connector.
SAS On-Demand
SAS On-Demand for real-time access to your new SAS datasets.
Custom Dashboards & Special Reports
Fusion Features and Benefits
- Connector.
Modular Configuration
Choose the features and modules that fit your study needs.
- Connector.
Real-Time Reporting
Live access to dozens of project reports to enhance study data visibility.
- Connector.
Real-Time Data Review
Live access to review, query and manage study data from any computer in the world and on any device.
- Connector.
Real-time Notifications
Real-time notifications for all personnel, total user control through the Communications Portal.
- Connector.
24/7 Real-Time Support
Outstanding site and PM/Monitoring Customer Care personnel available up to 24/7.
- Connector.
Configurable report builder
Use Axiom’s dynamic Report Builder to quickly access study data to build your own reports. Access your entire eCRF with drag and drop tools to create desired reports.
- Connector.
Rapid eCRF / Study Deployments
Rapidly configured & deployed in weeks by Axiom personnel, first view of your database generally within 10 days.
- Connector.
Project Portal
Access all of your study’s documents directly within Fusion.
- Connector.
Complete eCRFs
Fusion ensures that all mandatory fields are completed by site personnel. You will never miss key data.
- Connector.
Complex eCRF Logic
Powerful business rules result in a smart, well designed eCRF to capture your study data.
- Connector.
Inventory Tracking
Optional Inventory Supply Tracking Module manages all aspects of your study’s IP and ancillary supply reconciliation / tracking.
- Connector.
Con Meds Tracking
Track all study con meds. Directly link them to related AE/SAEs. Avoid double data entry.
- Connector.
Data Export
Access and export your clinical data on-demand via a web-based portal. User selectable file export formats. Choose to export all study data, or a sub-set of data.
- Connector.
Axiom EDC Cloud
Axiom Fusion is hosted in Axiom’s secure, triple redundant EDC Cloud. US northeast, west coast, EU, and Asian locations
- Connector.
21 CFR Part 11 Compliant
Fully compliant, secure, robust EDC solutions.
- Connector.
Integrated Help
Help tools, tips, how-to videos, eCRF Study Guides and up to 24/7 Customer Care.
- Connector.
No change orders
Unlimited database changes during study configuration allows you to focus on the study design.
- Connector.
Projected Subject Visit Reporting
Tools to assist coordinators and monitors with subjects’ pending visit scheduling.
- Connector.
Axiom eCRF / Reports Services Credit
Axiom offers a post-study launch services credit (a portion of the full study cost) as a draw-down account for your use. This credit is used to address changes through the operational phase at no cost to you.
Use Fusion to accelerate your next study!
+ The latest Fusion features and upgrades
+ Upcoming industry events and company news
+ Early access to our latest white papers, case studies and webinars