Benefits of a Unified eClinical Suite.
SEPARATE SYSTEMS
Do you find yourself chasing down your own study data and trying to reconcile them between multiple separate vendors and systems?
Are you settling for a less than efficient solution?
 Is your study still on separate systems?
Is your study still on separate systems?TRULY UNIFIED SYSTEM
A truly unified eClinical solution gives you single sign-on access to all your real-time study data, centralized.
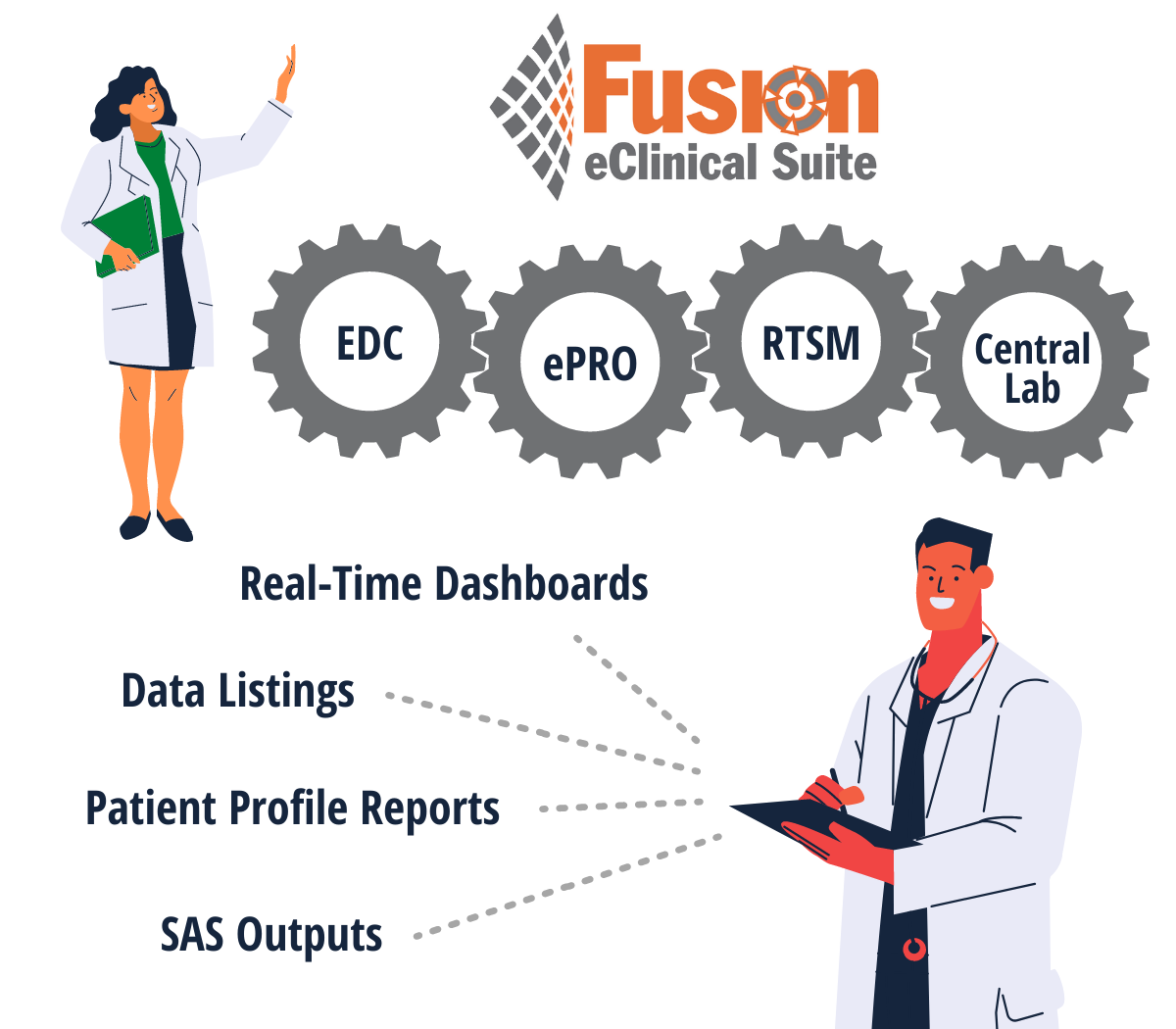
5 Benefits of a Unified Platform
With a Unified eClinical Platform you can empower your organization with data-driven studies on a centralized system that delivers faster and smarter results!
Ready to find out more? Book a demo today!
Our Mission: Drive Collaboration via Real-Time Actionable Data
Separate vs Truly Unified System
SEPARATE SYSTEMS
Lack of Integrated Dashboards
Critical data is stored across multiple systems.
TRULY UNIFIED SYSTEM
Data Fully Integrated on One Platform
Centralized data reporting on critical study factors.
SEPARATE SYSTEMS
Delayed Data Reporting
Study data may not sync in real-time.
TRULY UNIFIED SYSTEM
Real-Time Data Reports
All of your real-time study data, on demand, 24/7.
SEPARATE SYSTEMS
Slower Study Start-Up and Close-Out
Potential delays in start-up and close-out.
TRULY UNIFIED SYSTEM
Faster Study Start-Up and Close-Out
Time to first person in is reduced.
SEPARATE SYSTEMS
Extra Costs and Resources
Manual processes and managing multiple tools.
TRULY UNIFIED SYSTEM
Lower Costs and Resources
Fully integrated platform with a single sign-on.
SEPARATE SYSTEMS
Manual Tasks
Valuable time spent on manual reconciliation.
TRULY UNIFIED SYSTEM
Automated Tasks
Tasks are executed automatically across modules.

The Truly Unified Fusion eClinical Suite
Discover Fusion ModulesBetter decisions. Shorter timelines.
Unified Technology. Unmatched Service.