Axiom is your eClinical partner of choice.
Axiom brings together best-in-class technology and managed services to accelerate your study.
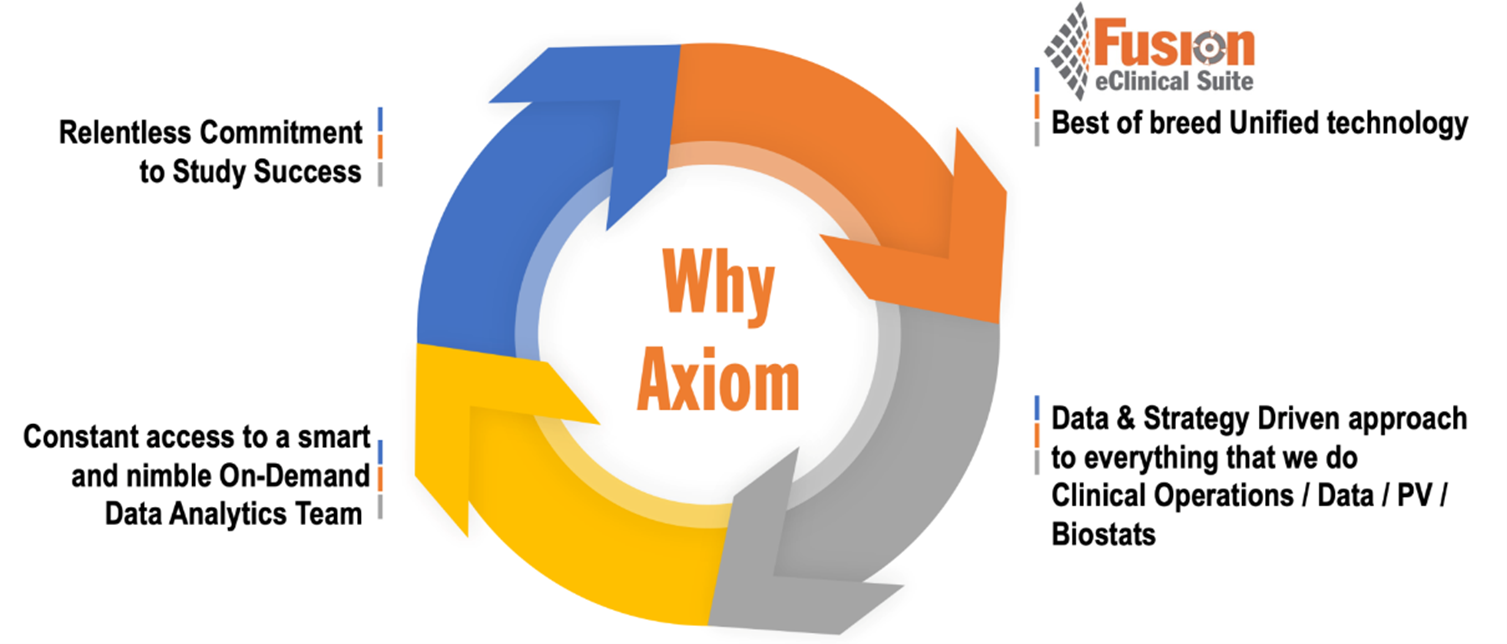
Why work with Axiom?
Key differentiators that put Axiom and the Fusion platform ahead of the crowd.
LEARN MORE
Focused on Small to Medium
We understand the unique challenges and needs of your organization.
LEARN MORE
Why work with Axiom?
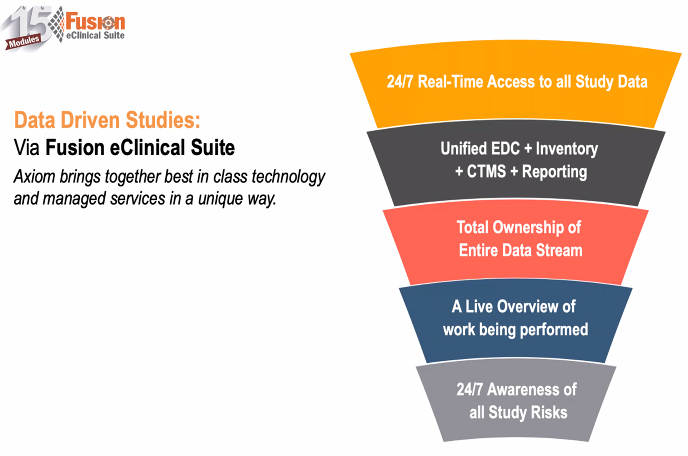
“Know More. Know It Sooner. Act Faster.” Technology should empower you to know more, know it sooner, and act faster. That’s what good technology does.

75+ Real-Time PM Reports
Live access to clinical data reports, DSMB reporting and data listings. Getting access to the status of your study shouldn’t require a PhD in computer science. Real-time study reports in 2 clicks.
Stop Manual Reconciliation
Axiom’s unified eClinical solutions means your EDC, Randomization, Drug / Device Tracking,Adjudication, Imaging and SAE / Safety data is in the same place.

Real-Time Study Activity Notifications
Key notifications about all of the information progress and issues within your study delivered 24/7 to your smartphone or e-mail.
Single Sign-On Access
Manage safety events in Fusion, no need for a separate safety database. How about managing your AE / SAEs / SADEs and overall safety reporting from within Fusion? Track events. Create narratives.
Integrated IWRS & Randomization
Fully manage your randomization within Fusion eClinical Suite. No need to configure a separate randomization system. Fully integrated with Inventory Management for complete digital management of your IWRS/Inventory.

Drug / Device Supply Throughout Your Study
Global awareness of inventory activities, balances, and issues as well as notifications of key events including inventory levels, shipments, requests and pending activities. Stop managing your drug or device accountability in multiple systems.
One of the keys to study success is the focus of the organization on the study details, consideration for planning and strategic thinking that goes into the initiation of the study. Axiom’s Think / Deploy / Launch process ensures that a well thought-out process is the basis for planning your study.

Dedicated Customer Care
Axiom’s Customer Care team are extremely focused on ensuring that every single user in the study chain is supported throughout the entire study. Great support is linked to high-quality data. Support personnel who actually are trained on your study’s eCRF. Quick answers to end-user questions means that the study moves along quickly and efficiently.
Value for Your Investment
Expanding the technology footprint could cost more in the short term, but can greatly enhance the overall study, your real-time knowledge, key business decisions and lower costs in the long term.
Focused on Small to Medium
We know how critical intuitive, cost-effective, and unified eClinical solutions are to small to medium size life sciences companies. We are here to empower you to do more with fewer resources, a smaller team, and tighter budgets.

Axiom is an Embedded Partner to Your Team.
Axiom functions as an extension of the overall clinical study team, focused on the study’s progress, partnered through the challenges and the successes.

Configure Fusion for Your Unique Study Needs.
Clinical data is at the heart of your project. Work with a highly configurable, fully integrated eClinical partner so you can focus on the research, not the tech behind it.
