When do you start preparing your TMF (Trial Master File)? What tools can you use to ensure your clinical study teams have secured and real-time access to all of your essential TMF documents? Axiom’s unified eClinical Suite Fusion lets you upload and manage all your documents with ease and offers study-specific metadata support.
As many activities occur at the beginning of a clinical study, a considerable amount of documentation would be generated and filed on TMF. But as the study goes along, have you run into issues since the documentation owner became less pronounced? Have you noticed that responsible individuals on the clinical study get busy with actual products but then forget to document the items to go along with the process?

The documentation contained within the TMF needs to be sufficient to reconstruct the trial activities and key decisions made along with the trial.
This article will briefly recap the presentation from Axiom’s Heather DiFruscia, Associate Director of RTSM/IWRS, and Brian Dempster, Senior Director of Global Clinical Management. In their session, they shared some of the common FDA findings regarding documentation and how to take advantage of people and technology to tackle related challenges.
Their presentation covered the following topics:
- Failure to prepare is preparing to fail
- Lack of documentation
- Documentation not contemporaneous
- Lack of documented process
- Start with the end in mind
- Choose a TMF reference model
- Assign documentation roles and responsibilities to specific teams
- Map out TMF documents and milestones with clear timelines
- Translate your plan to workable technology
- Ask the Fusion Team
- What are the challenges in transitioning from paper to an eTMF?
- How do you manage documents stored with other vendors?
- What modules have the biggest impact from a documentation standpoint?
- What kind of end-user support and resources are available for eTMF?
- Ready to find out more about Fusion?
Failure to prepare is preparing to fail
A lot of common FDA audit findings are about documentation. Unfortunately, documentation often becomes an afterthought in clinical studies. Study teams would think documentation takes care of itself when works proceed. It indicates that the absence of planning and the nuanced failures to document activities as the trial progresses could eventually lead to FDA audit findings.
Lack of documentation
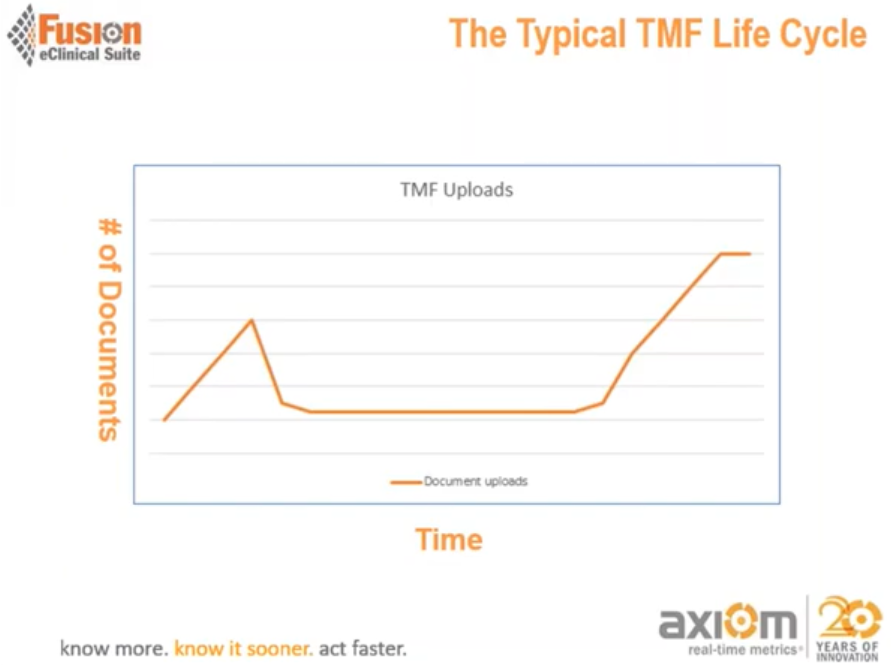
The typical TMF life cycle shows the number of documents generated and filed on TMF peaked at the beginning of a study but gradually decrease as the study carries on. The plateau indicates a lack of documentation along with the study activities and implicates the study needs to have precise planning to prevent the documentation from becoming an afterthought.
Documentation not contemporaneous
You could probably recall what you did at 9 AM today with a great deal of accuracy and be able to illustrate that. But will you be able to do the same if the question is, “What were you doing four years ago today at 9:00 AM?”
Compared to recording documents on time to reflect the study activities accurately, it is almost impossible to have the same quality and accuracy if the documents are loaded at the backend of the study, as shown in the typical TMF life cycle.
Lack of documented process
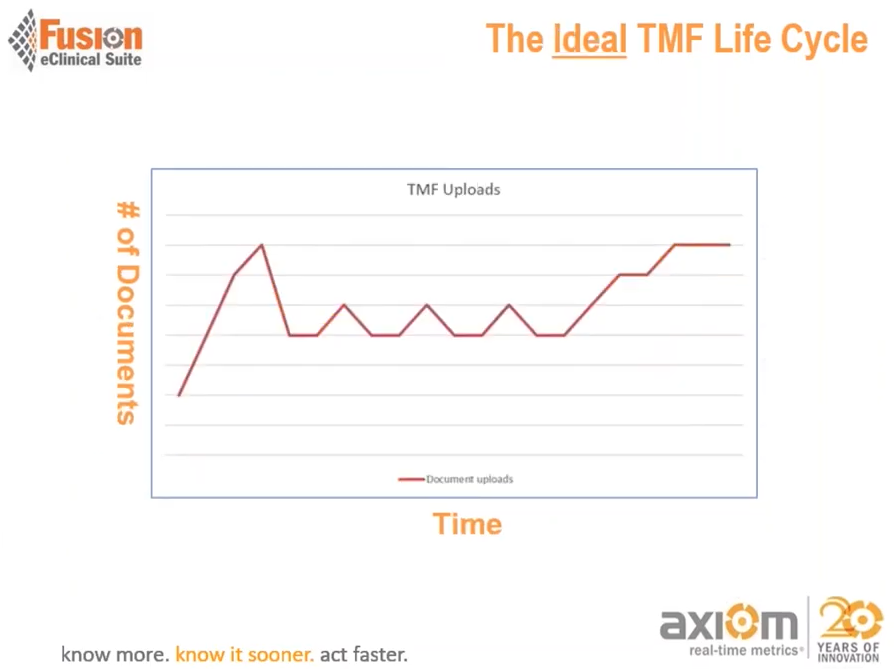
As the study continues, rather than leaving the documentation behind and assume the owner on documentation will catch up with the process, you would want to see documents kept being collected and filed on an ongoing basis throughout the study. In this way, your study team can keep track of the documentation while the workflow remains contemporary with the documentation.
It is essential to understand the purpose of having contemporaneous documentation is to show regulators and inspectors in the study that you can reconstruct the trial activities. The significance of having a documented process is that you can display in an inspection that you complied with regulatory requirements throughout the study.
In conclusion, do not just start collecting documents — plan for the issues and the process with the documentation plan.
Start with the end in mind
At Axiom, we believe in starting with the end in mind. Therefore, a clear plan at the outset of the study is vital.
Choose a TMF reference model.
Every study should begin with a TMF reference model mapped out. Then, you can take the reference model and decide how you will map that model to the requirements of your specific study. The TMF reference model provides standardized taxonomy and metadata and outlines a reference definition of TMF content using standard nomenclature.
Assign documentation roles and responsibilities to specific teams.
An eTMF system like Axiom’s Fusion allows you to keep the activities of responsible parties visible within the eTMF and enforce accountability since you can custom user permissions for individual files and folders. In this way, relevant documentation owners can work with their teams, knowing the responsible parties in the eTMF. Ultimately, the TMF should always be audit-ready, complete in chronological order, and accurate enough to portray a study’s conduct as a whole process so that the study is valid with the integrity of the data.
Map out TMF documents and milestones with clear timelines.
It is crucial to bring the people, the process, and technology into the clinical study documentation with clear timelines so that you can keep track of documents and their approval statuses and promptly inform responsible parties with a list of action items and user-level notifications.
Translate your plan to workable technology.
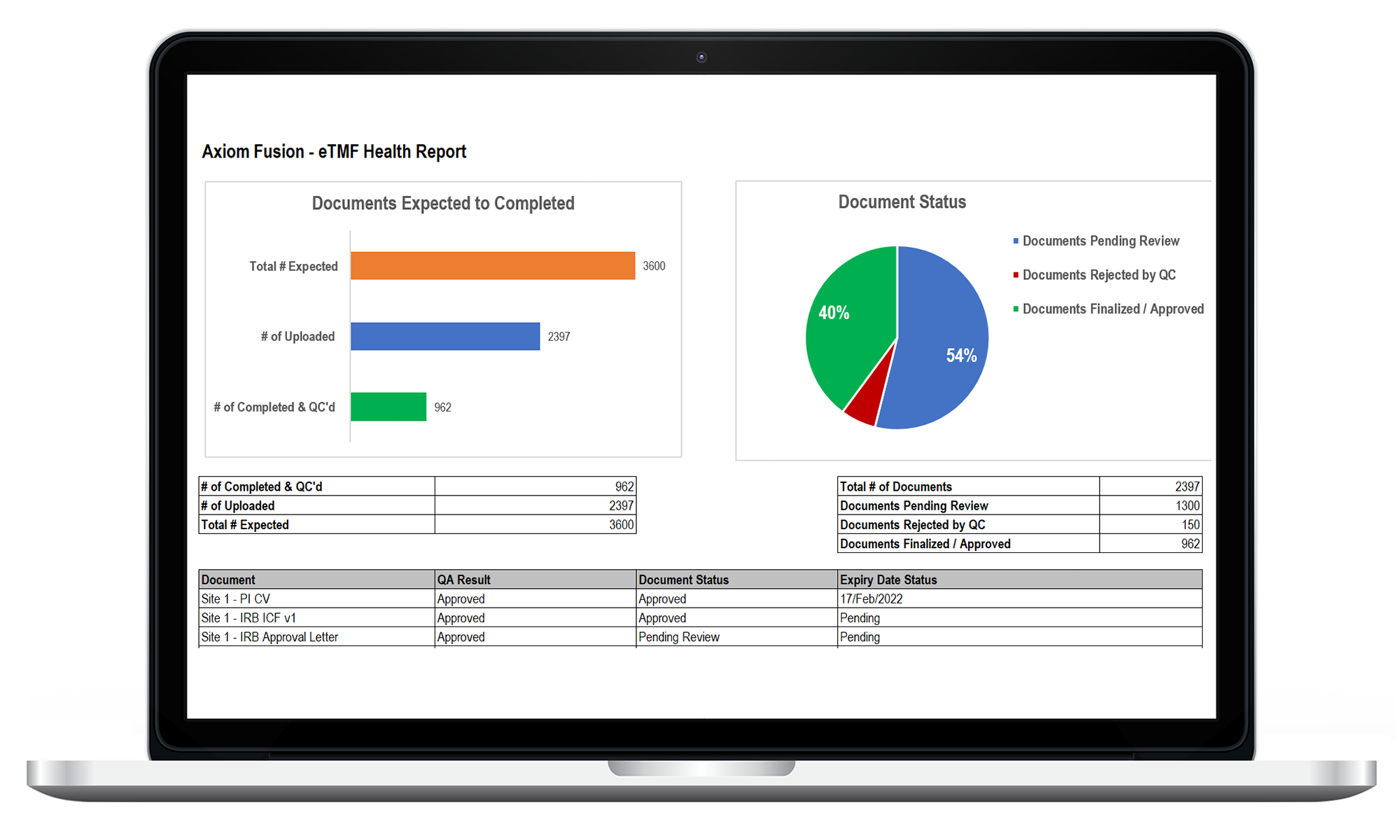
Fusion eTMF enables you to have real-time access to upload and manage all essential TMF documents with a secured single sign-on. In addition, built-in features such as eTMF Health Reporting feature that monitors and sends timely notifications on overdue or missing documentation, supporting an intuitive and smart solution for managing your TMF.
Additionally, other highly configurable modules and technology available on Fusion, such as CTM Tracking, can also benefit your study. For example, it allows you to track bulk or device inventory from the supplier to the depot, site, or the subjects, empowering you to know more, know it sooner, and act faster to meet your project’s unique needs.
In conclusion, having a thorough plan and translating it into workable technology such as Fusion also means you could take advantage of other robust modules from the unified platform to run your study.

Ask the Fusion Team
Do you have more questions about eTMF? Brian Dempster, Axiom’s Senior Director of Global Clinical Management is here to answer your questions.
Question: What are the challenges in transitioning from paper to an eTMF?
Brian: The logistics are becoming less of an issue and straightforward. It is the validation that is challenging. If you transition from paper to an eTMF, you need to plan how to do so and what kind of processes you will follow. Another challenge is to plan what you will do with the paper once the transition is completed.
Question: How do you manage documents stored with other vendors?
Brian: You need to ask yourself questions such as: what are the activities that vendors have taken? What are they doing to the documents during activities? Do you have a document representation of those activities? TMF is not a single location entity and can be spread to many different places.
However, it would be best to have the basics moulded into your plans: documents you expect to see, responsible for the documents, and a check-in mechanism ensuring those documents are created and filed at the milestone is a must-have. Bring your vendor into your TMF planning and ensure you have clarity between you and your vendor. At last, you should have regular transfers set up of those documents to a location where you can view and store them.
Question: What modules have the biggest impact from a documentation standpoint?
Heather: eTMF module and CTM tracking modules, along with other modules such as eConsent, eSource, and ePRO, are all critical. Using these modules means that you have awareness, visibility, and traceability at all levels. When you use a unified platform with multiple modules with real-time notifications, all your data entry is in one place.
Question: What kind of end-user support and resources are available for eTMF?
Brain: Axiom’s dedicated 24/7 customer care team. The team is trained on all the details of clients’ unique studies (eCRFs, Fusion configurations, etc.) and dedicated to ensuring a five-star end-user experience. Whether it’s a coordinator from a study team working with Fusion EDC or a patient using Fusion ePRO, Axiom’s customer care team is always ready to provide one-on-one, real-time support.
Ready to find out more about Fusion?
Get in touch with our team and book a demo today!