Fusion ePRO Tools
Learn how adding ePRO to your study can increase ease of use, improve compliance, decrease Site burden, and maintain data integrity.
The Fusion Advantage
Ready to find out more? Book a demo today!Features and Benefits
ePRO for All Devices
Multiple options for Subjects to submit their ePRO entries.
LEARN MORE
Real-Time Data
Timely, quality, actionable data at your fingertips.
LEARN MORE
Accelerated Solutions
Get your ePRO-enabled study running in 30-60 days!
LEARN MORE
Exceptional User Experience
We make it easy for Subjects to participate and find support.
LEARN MORE
ePRO for All Devices
Multiple options for Subjects to submit their ePRO entries.
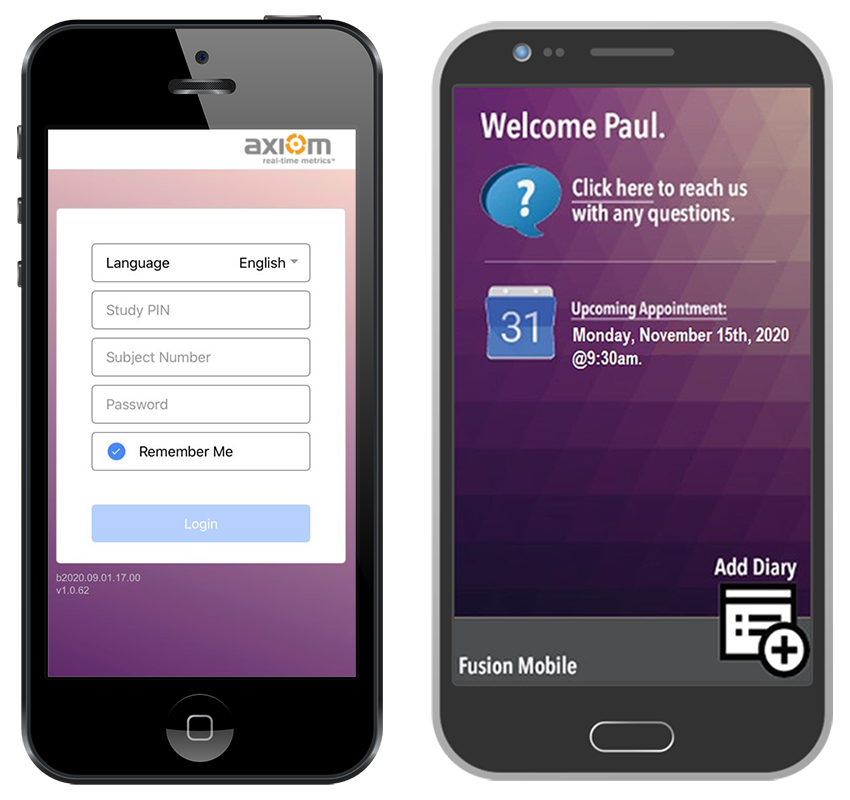
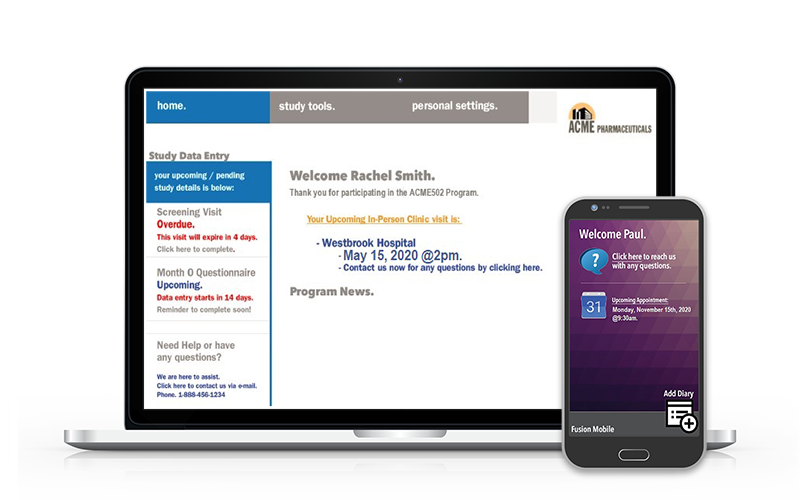
ePRO / eDiary Mobile App
iOS and Android compatible mobile app.
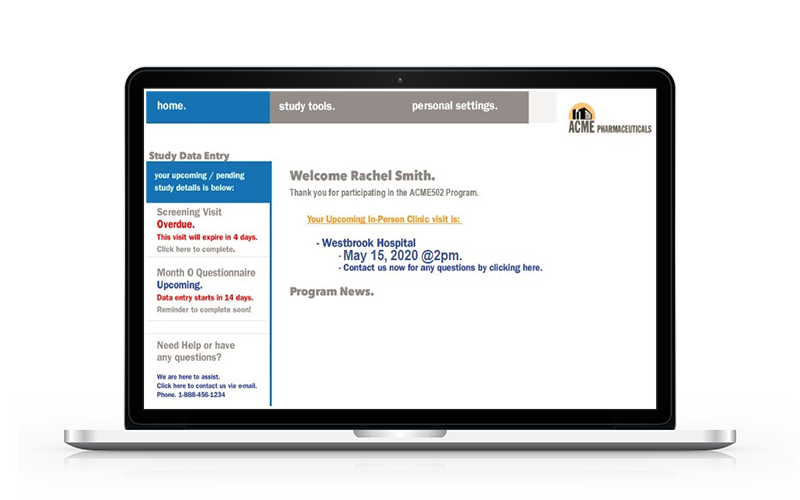
ePRO / eDiary Patient Web Portal
Compatible on all web browsers.

ePRO / eDiary Phone/IVRS
Call-in diary entries and questionnaires.
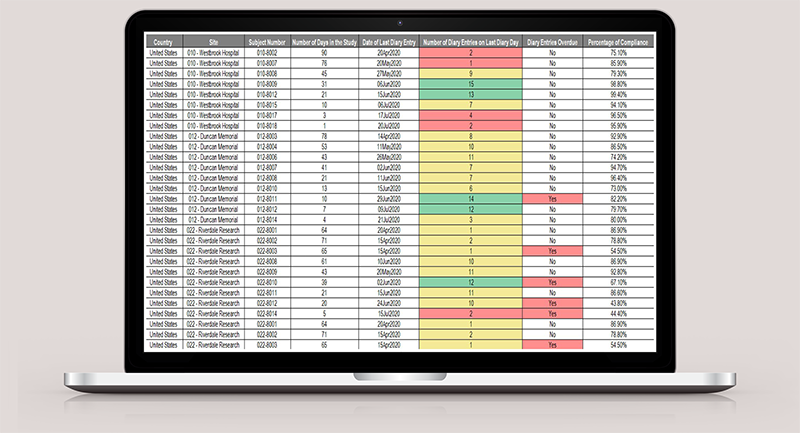
Real-Time Data
Timely, quality, actionable data at your fingertips.

Automatic Data Sync
Real-time syncing to Fusion database.
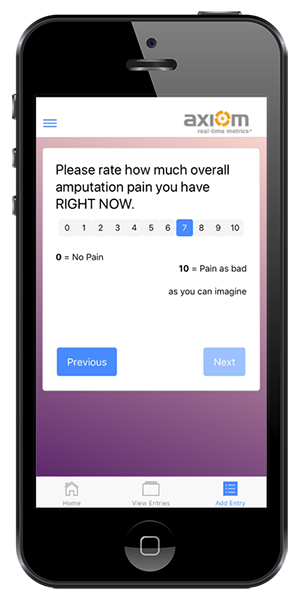
Improved Data Integrity
Reduce manual data entry errors and save time.
Accelerated Solutions
Get your ePRO-enabled study running in 30-60 days!

Turnkey Device Service
Ready-to-use device kits.

Fusion ePRO Template Libraries
Access to hundreds of standard questionnaires / assessments.*
* Sponsor requires a license to use applicable questionnaires – Axiom provides support with licensing process, if applicable
Exceptional User Experience
We make it easy for Subjects to participate and find support.

24/7 End-User Support
Our dedicated customer care team are here to help, anytime!
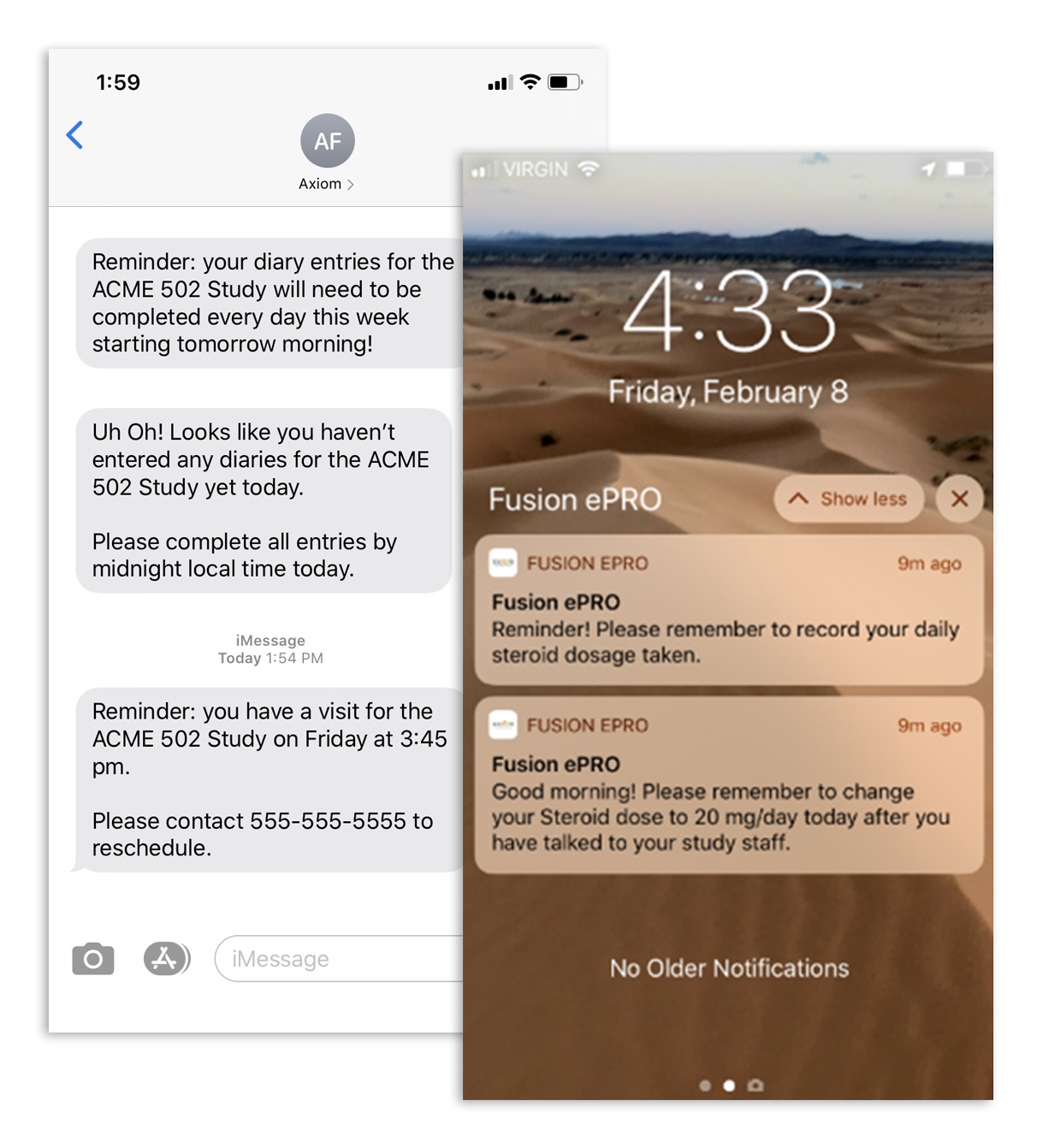
Patient Notifications
Automatic reminder notifications to encourage Subject compliance.
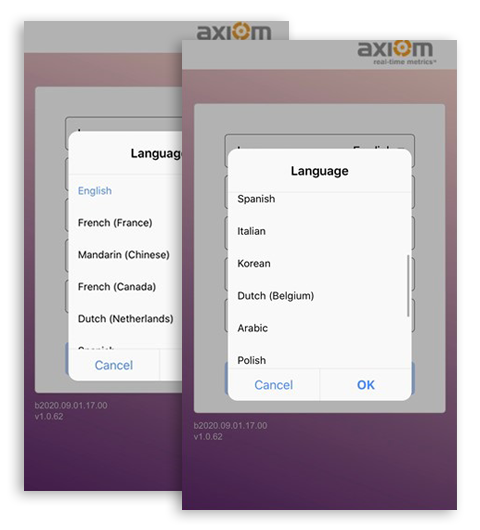
User-Friendly Design
Simplified features for End User Success.

ePRO and Fusion eClinical Suite
Discover More Fusion ModulesBetter decisions. Shorter timelines.
Unified Technology. Unmatched Service.

Talk to us about ePRO for your next study!
+ The latest Fusion features and upgrades
+ Upcoming industry events and company news
+ Early access to our latest white papers, case studies and webinars